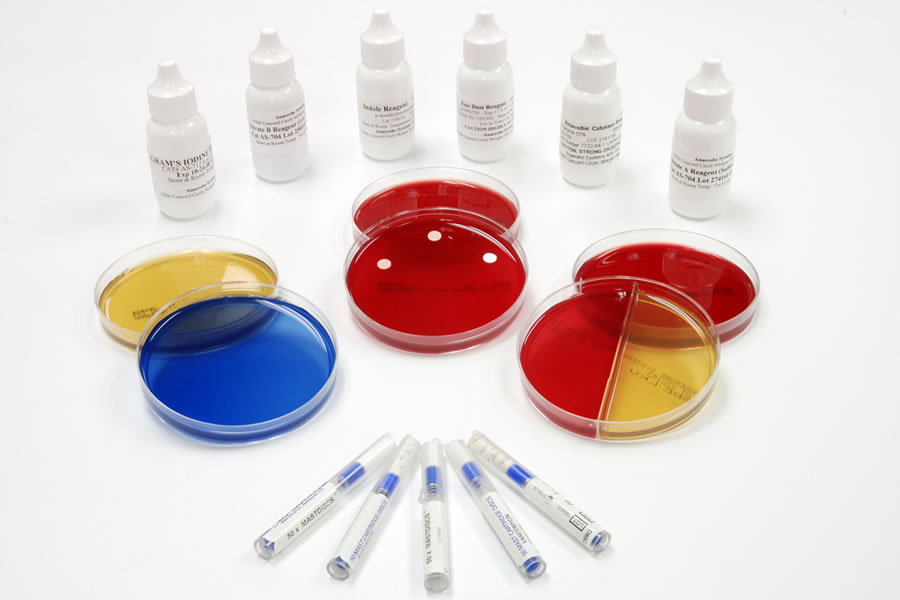
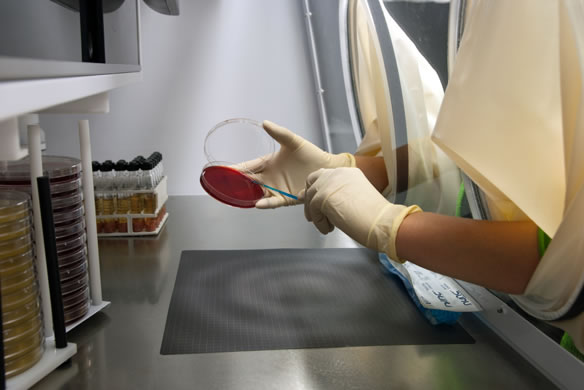
Anaerobe Systems has over 40 years of experience in growing and cultivating anaerobic organisms using anaerobic chambers. Our anaerobic chambers are developed and designed with direct microbiologist input. We are a unique anaerobic chamber manufacturer in that our own microbiologists work in our chambers every day to validate the culture media we produce.
- Designed with direct microbiologist input
- On-site installation & training always included with purchase
- 2.5 day hands on training always included with purchase
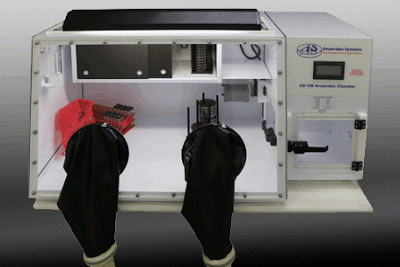
Our AS-150 anaerobic chamber is an entry level, lower cost small chamber with fewer features and less workspace, but with a lower cost and smaller footprint. The AS-150 is ideal for smaller labs that typically have fewer than 100 plates in their incubator, or labs that don’t have the space or budget for a larger chamber.
OurAS-500 gloveless anaerobic chamber is a large chamber with many added features to improve ergonomics, reduce daily maintenance, and monitor & control the anaerobic environment. The high capacity incubator and large work floor are ideal for large clinical or research labs, research studies, or bio-therapeutic development. The unit requires minimal daily maintenance, and working inside the chamber is quick and easy compared to other gloveless models. The AS-500 has a robust environmental conditioning and monitoring system which includes HEPA and chemical filtration, oxygen monitoring, humidity removal, and oxygen removal.